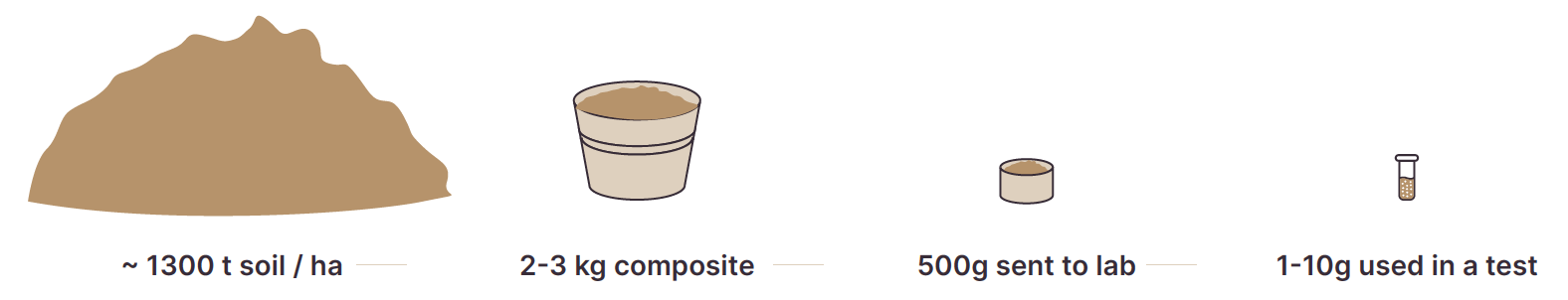
- Soil testing is a vital tool to identify issues affecting profitable crop and pasture production. Knowing how to best manage the soil means first understanding soil chemistry, physics and biology.
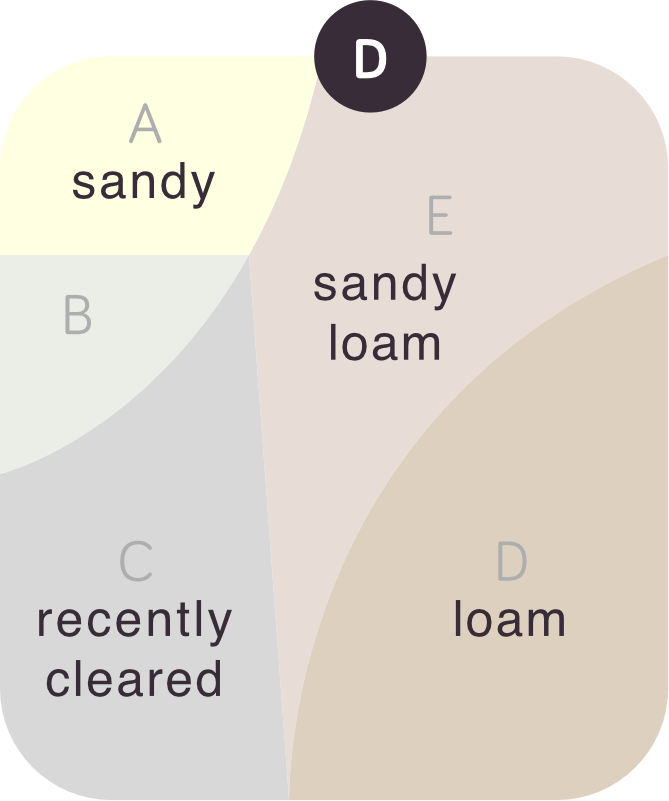
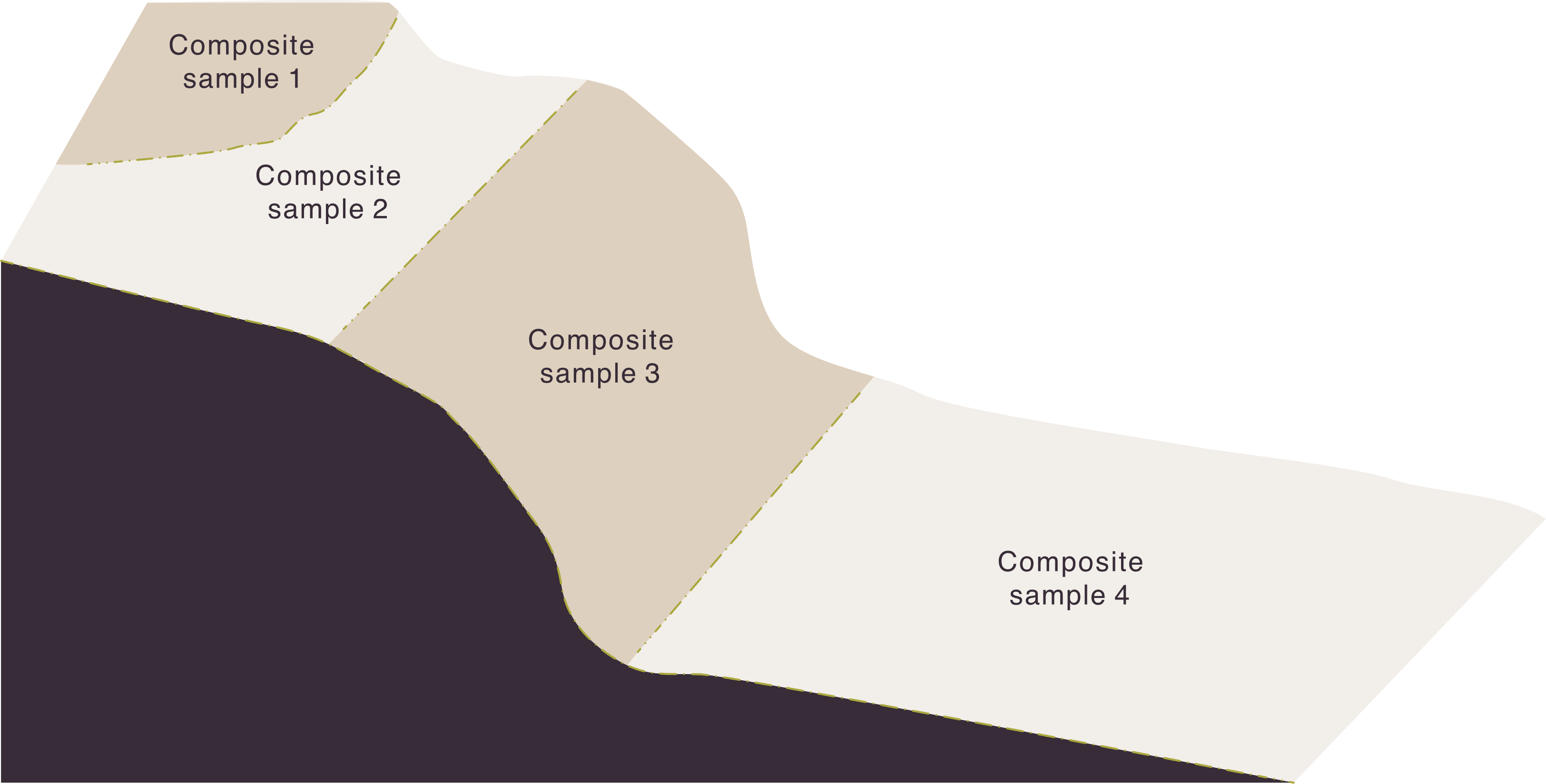
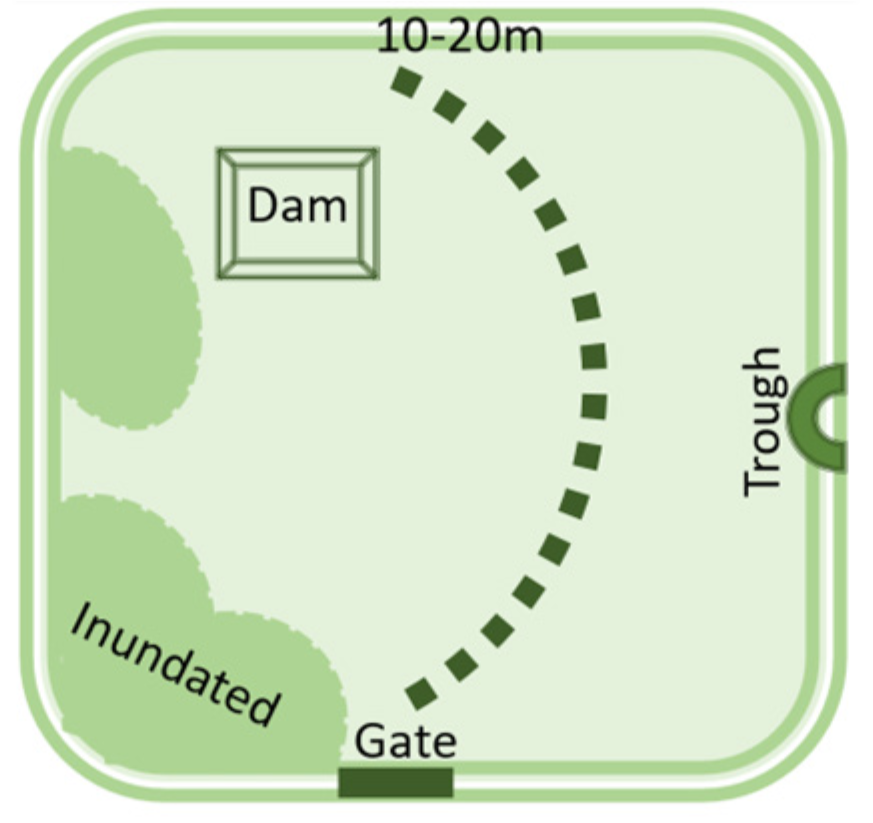
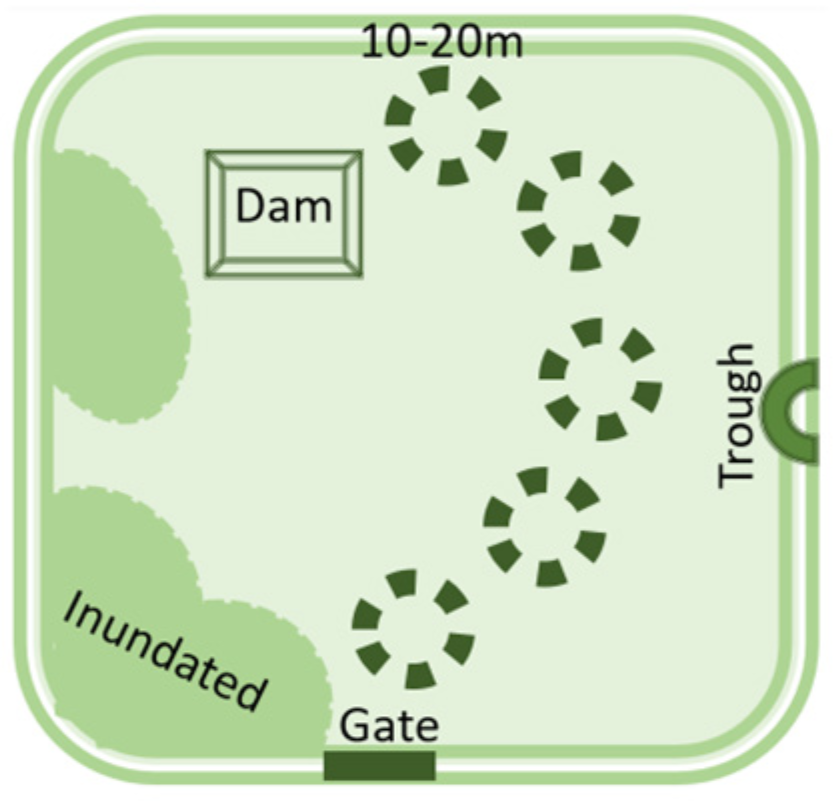
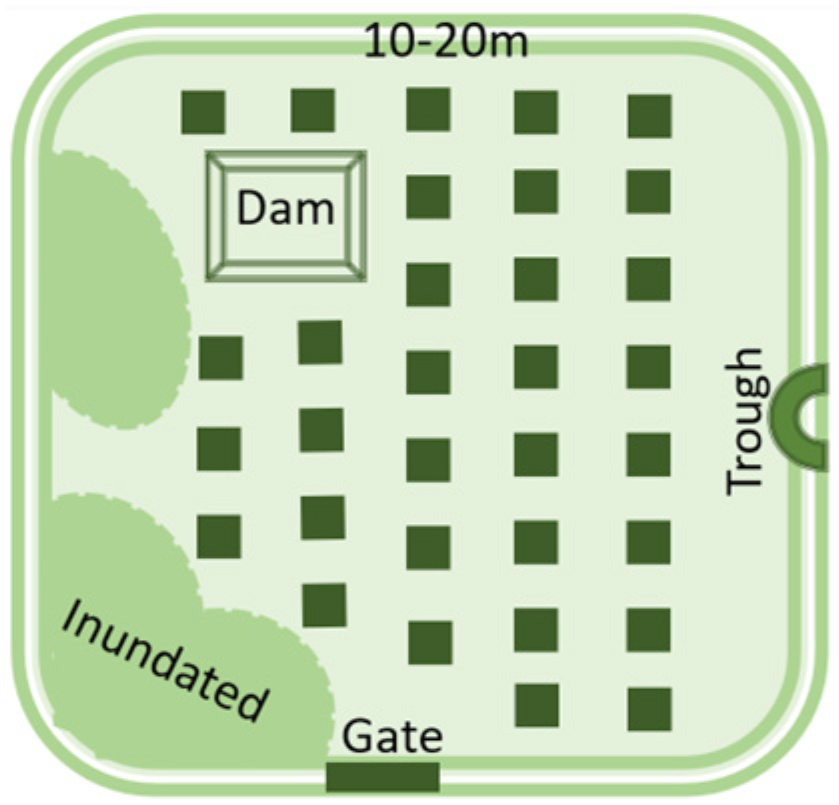
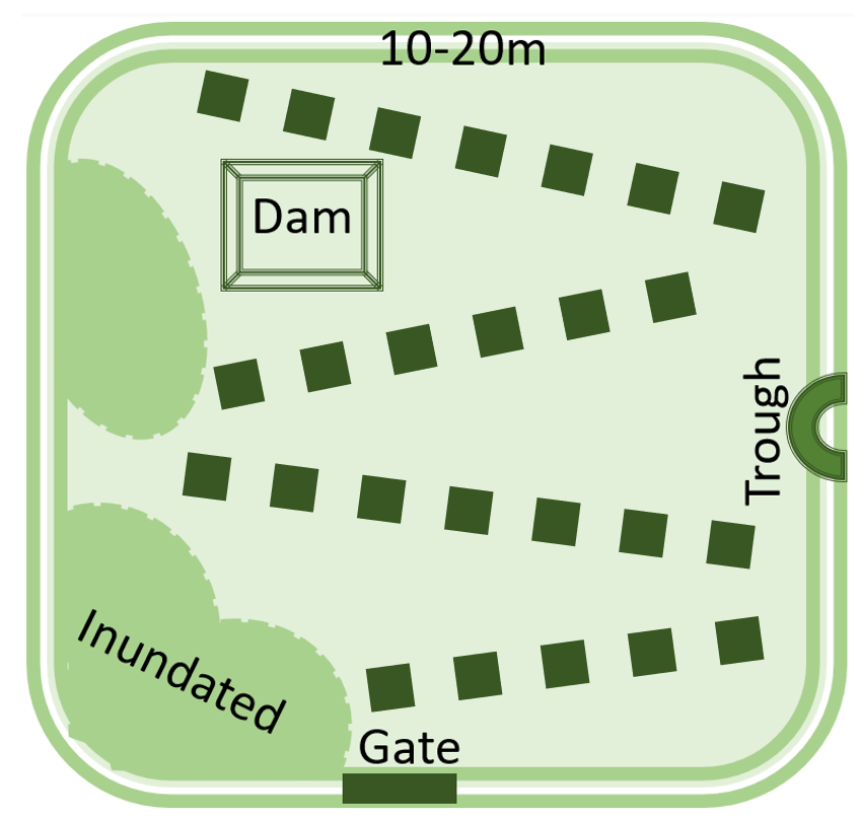
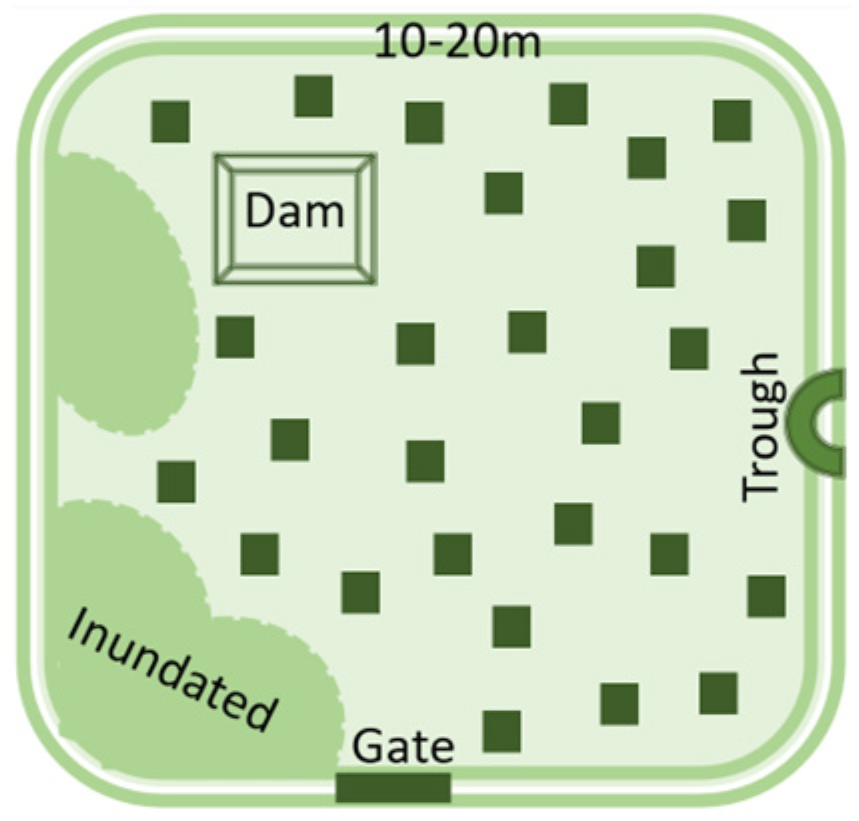
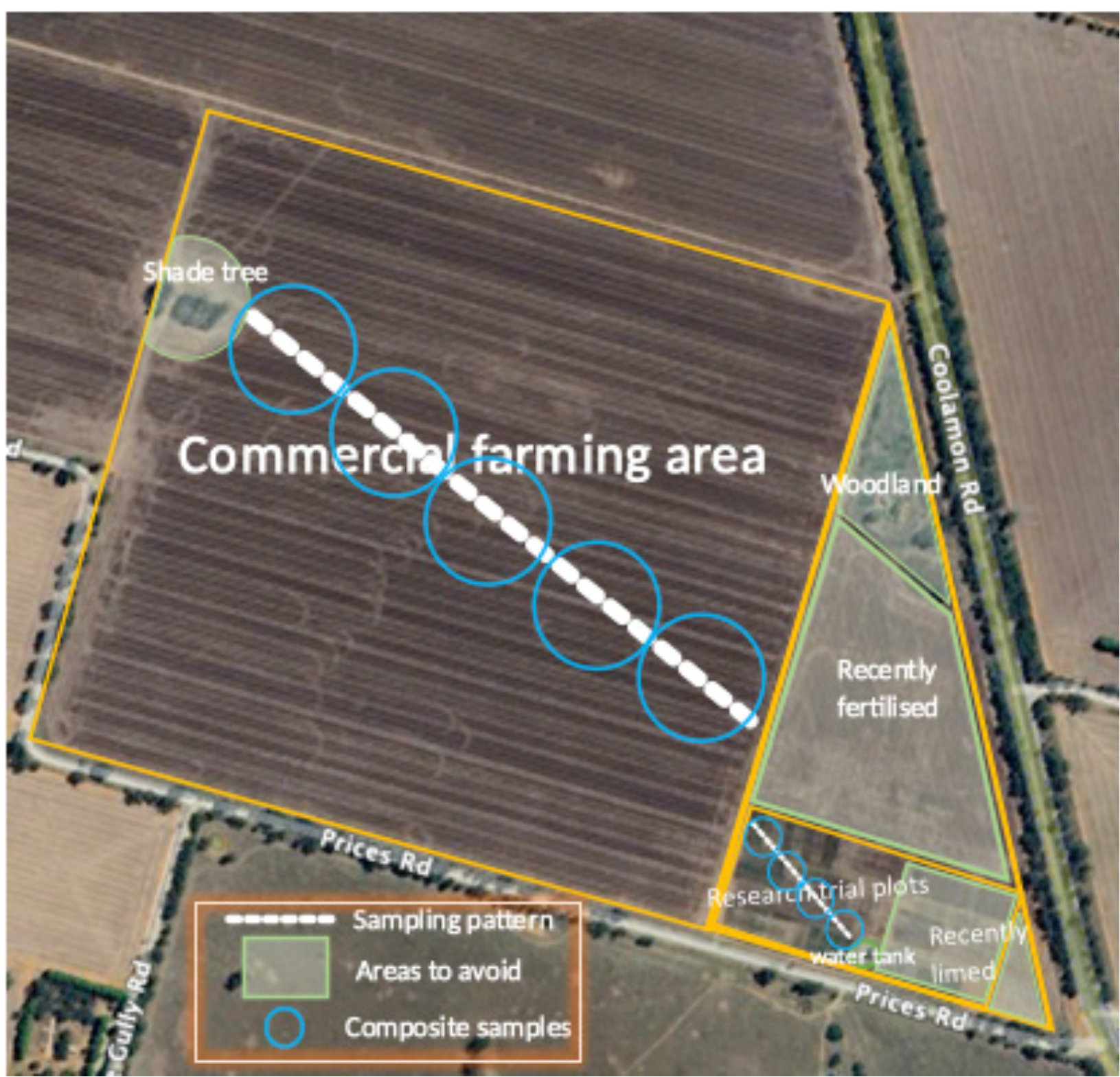
- Soil is inherently diverse and farm management practices add to the diversity. For example, no-till and precision fertiliser placement lead to nutrient bands in the soil, rather than evenly distributed nutrients.
- Understanding soil nutrient stocks is critical to best practice crop nutrition.
- Fertiliser planning. Which nutrients are needed and how much?
- Diagnosing a problem. Is a soil issue the reason behind poor crop or pasture growth?
- Monitoring. How soil properties are changing over time.
- Compliance. Does the status of soil in farm meet required environmental standards.

The 4Rs of good
fertiliser management.
> nutrientstewardship.org/4rs