
Many results need interpreting with context, so it is not possible to give an ideal range for each nutrient. For example, a ‘low’
potassium result is different between canola and wheat and will change depending on the soil type and season. Your local agronomist is the ideal person to interpret soil nutrient results and calculate nutrient application rates for your situation.
Some laboratories will give guidelines or ideal ranges. Interpret these with care as they might not be relevant to your situation.
Always check the units and test method when interpreting results.
mg/L = milligrams per litre
meq/100g = milli-equivalent per 100 gram of soil
meq % = milli-equivalent percent cmol/kg =
centimole per kilogram; also written as cmol+ kg-1
ppm = parts per million
μS/cm = microSiemens per centimetre
mS/cm = milliSiemens per centimetre
dS/m = deciSiemens per meter
ha = hectares
t = tonnes
1 mg/kg = 1 ppm = 1 mg/g
1 meq/100g = 1 meq % = 1 cmol/kg = 1 ppm
1 ha = 2.5 acres
1 t = 1000 kilograms
1 deciSiemens/metre (dS/m) = 1
milliSiemens/centimetre (mS/cm) = 1000
microSiemens/cm (μS/cm)
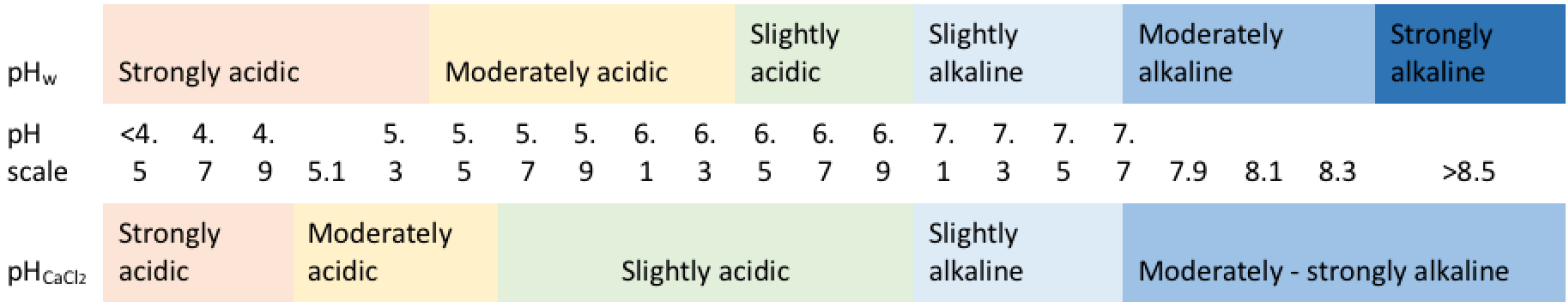
Soil pH is a measure of acidity or alkalinity. pH is measured in either water or calcium chloride (pHCaCl2). Check whether pH is listed as pHw or pHCaCl2. Some laboratories will include both. The two methods give slightly different readings.
pHw = pHCaCl2+ (0.5)

The pH of this soil sample is ideal for most crops, but too high for acid-loving crops. There should not be any nutrient toxicities or deficiencies induced by pH.
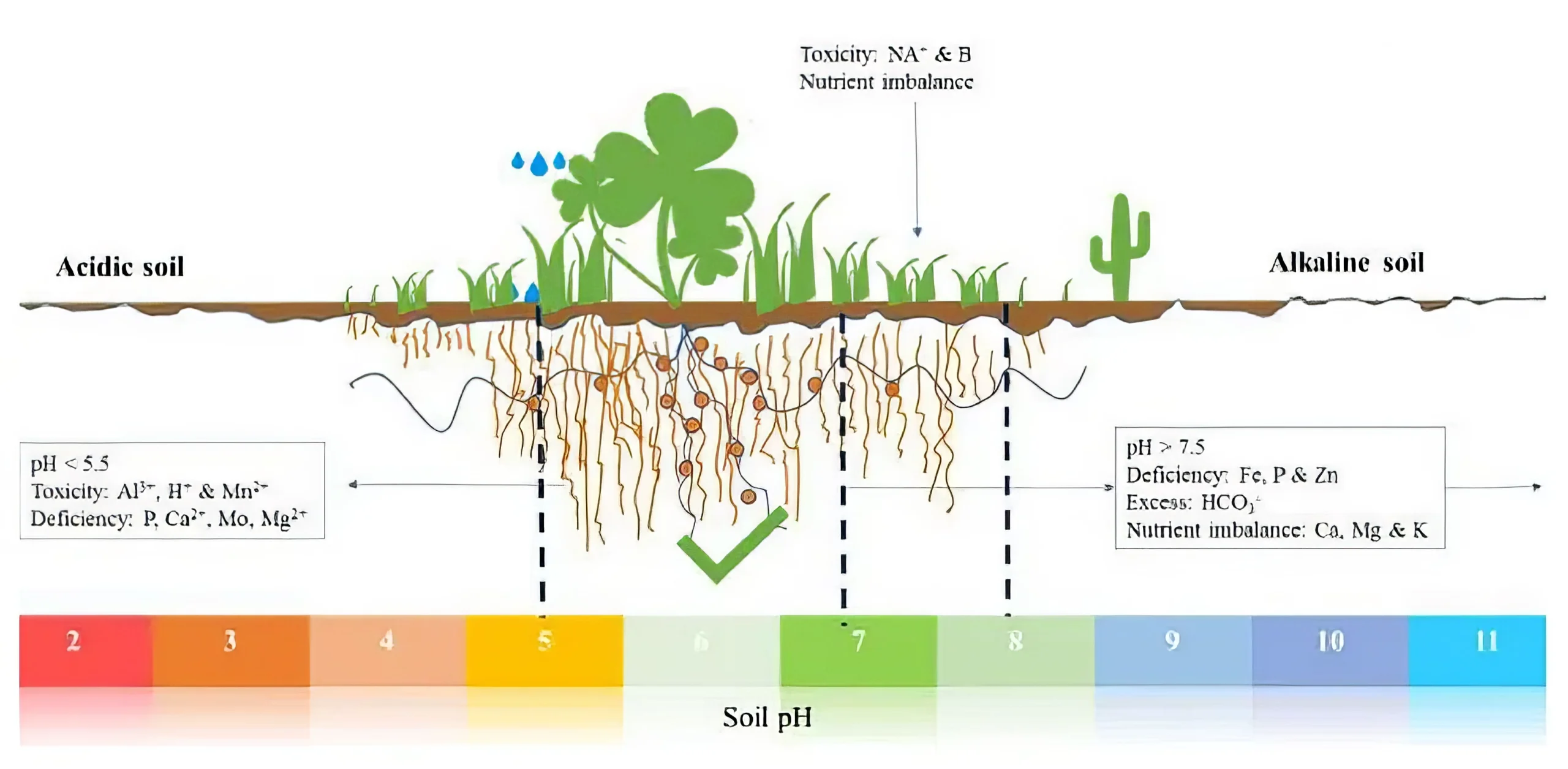
Figure 1. The effect of soil pH on nutrient availability, toxicities, deficiencies, and imbalances.
Source: Msimbira & Smith, 2020.
Issues with...
Electrical conductivity (EC) is a measure of soil salinity. The more salts in the soil solution, the higher the EC reading.
Electrical conductivity (EC) is a measure of soil salinity. The more salts in the soil solution, the higher the EC reading.
Gypsum can elevate salinity results.
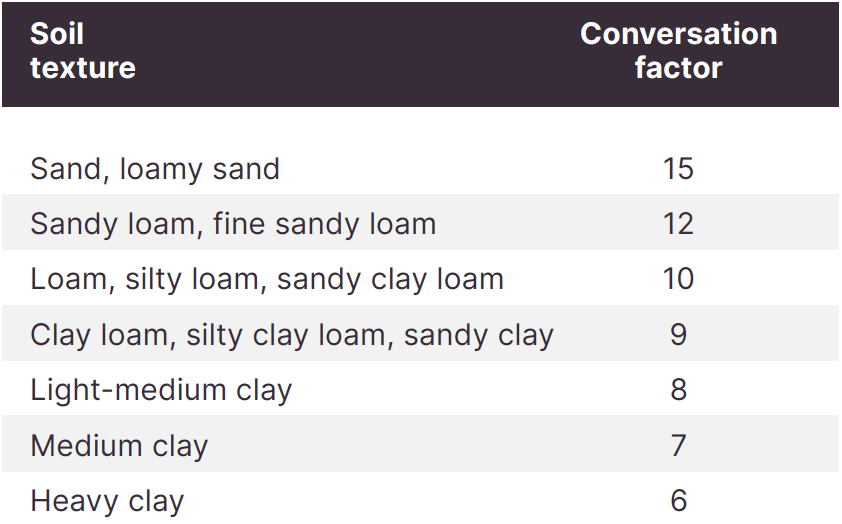
EC1:5 is measured by mixing 1 part soil to 5 parts water, shaking the sample, filtering out the solids, then measuring the salinity of the water. This EC value does not directly represent the salinity of the soil. Soil texture influences how salt levels affect plant growth. EC1:5 values are multiplied by a texture factor to give a better idea of soil salinity and presented as ECe.
ECe uses the 1:5 measurement multiplied by a soil texture factor (see Table 1).
ECse (EC saturated extract) directly measures salinity the saturated extract of a soil sample. This method is less common because it is time consuming and expensive.
Table 1. Conversation factors from EC1:5 to ECe.
Check the units (dS/m, mS/m, or uS/m) and method when interpreting results. Most soil salinity measurements are presented in deciSiemens per metre (dS/m) but sometimes other units are used. Check both parts of the units – whether dS, mS or μS, and whether m or cm after the slash. The following table converts between the different units.
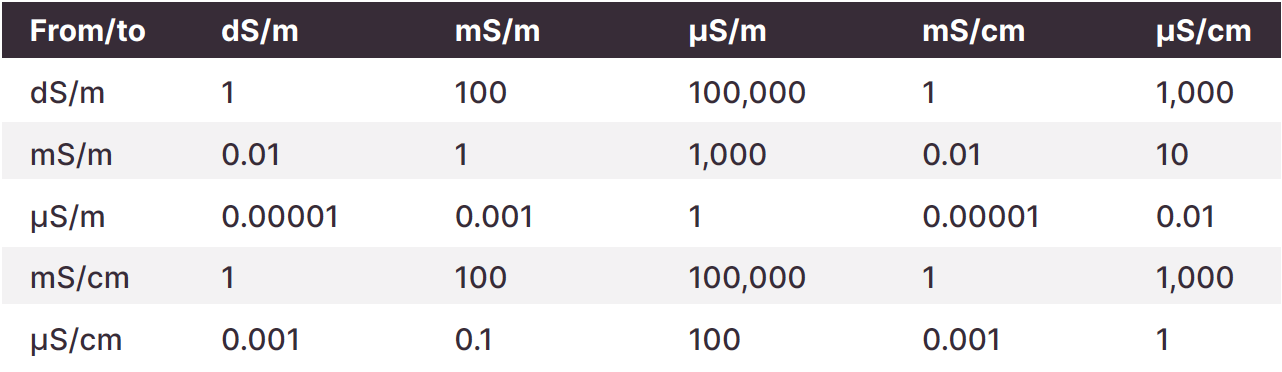
Table 2. Interpreting ECe
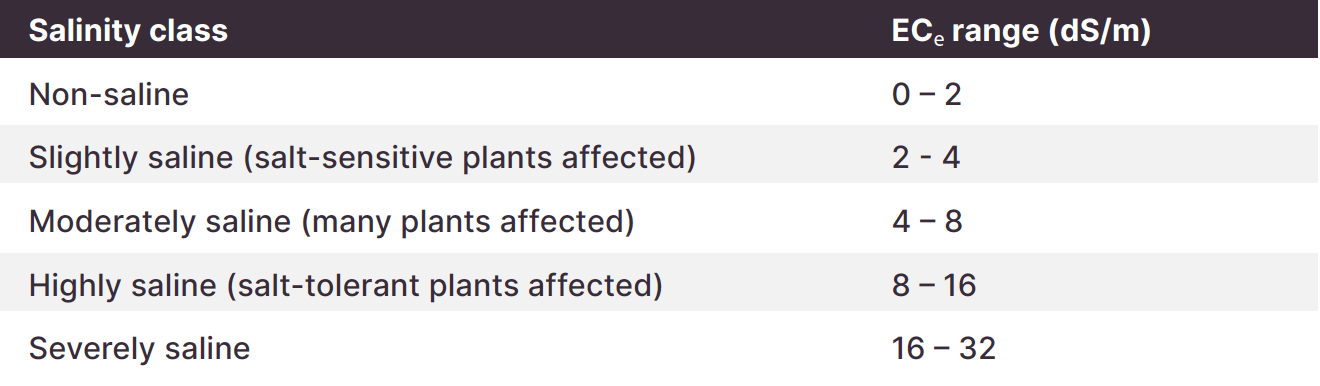

Salinity as denoted by EC. High chloride can be a sign of soil salinity. Interpret EC and chloride results together.
Chloride results are presented as either mg/kg or ppm. Sometimes chloride is estimated by multiplying the EC value by 640.
Values above 120 mg/kg in sand to sandy loam, >180 mg/kg in loam to clay loam, and >300 mg/kg in clays can indicate a salinity issue.

EC and chloride levels are very low in this sample. Soil salinity is not a problem for crop growth.
Effective Cation Exchange Capacity (ECEC), Nitrogen. Organic matter is a key driver of soil ECEC (a measure of soil fertility). Nitrogen is released (mineralised) from organic matter throughout the season, providing a source of nitrogen to crops.
Plant material such as roots and leaves in the sample can inflate the result. Check the test method.
Ideal range: 1 – 2% (broadacre cropping), 2 – 5% (irrigated cropping, pastures in high rainfall zones). The desired range will vary based on soil texture, ECEC and rainfall zone, but generally <1% is very low. Lower rainfall areas, sandier soils (and therefore lower ECEC) will have less OM. Pastures in high rainfall zones could be expected to have 2 – 5% organic matter.
Organic matter (%) is estimated from organic carbon (%) tests. There are three main ways to measure soil organic carbon:
Labs measure organic carbon and convert the number to organic matter by multiplying by 1.72 or 1.75. E.g., if organic carbon = 1.45%, the organic matter content is 1.45 x 1.72 = 2.5%.
*Labs use slightly different conversion factors.
The test result page should list the conversion factor.

CEC varies depending on clay %, the type of clay, organic matter, and soil pH. Generally, the more clay and/or organic matter, the higher the CEC. Soils with a low CEC have a low resistance to changes in soil chemistry caused by land use.
pH, Soil dispersion. Acidic soils can have elevated exchangeable aluminium. Dispersive soils may have high (>5%) exchangeable sodium.
Results are presented as either cation exchange capacity or effective cation exchange capacity. The result is a sum of the percentages of exchangeable cations.
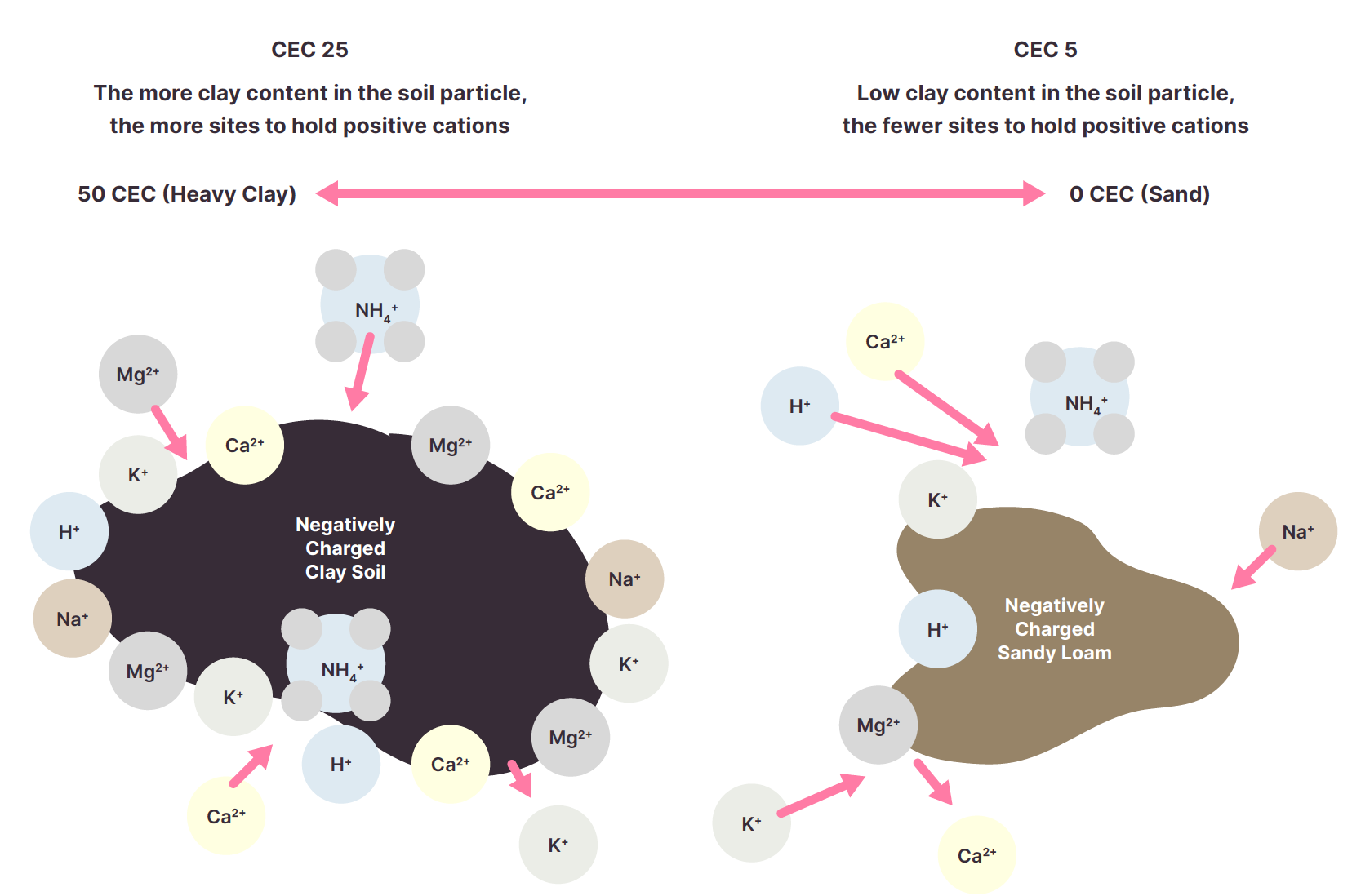
The most common cations in the soil are calcium (Ca2+), magnesium (Mg2+), potassium (K+), sodium (Na+) and aluminium (Al3+). They are often presented as a %, but some labs will also present kg/ha, ppm (mg/kg) and cmol+/kg.
If the soil test report does not provide exchangeable cation percent, you can calculate it:
(cmol+kg-1 / CEC) x 100.
For example, if potassium (K+) = 1.1 cmol+/kg and the ECEC is 7.5:
(1.1/7.5) x 100 = 14.6%.
Some laboratory results will give effective cation exchange capacity (ECEC). This calculation includes the alkalinity inducing cations Ca2+, Mg2+, K+, Na+ and acidity inducing cations H+ and Al3+. It is more accurate than CEC.
Modern tests use cmol+/kg as the unit of measurement. Older tests used meq/100 g or meq%, which is the same as cmol+/kg.
cmol+/kg = meq/100g = meq%
Ideal range: Varies depending on the soil. Low fertility soils have a CEC <5 cmol+/kg.
Ideal range: Varies depending on the soil. Low fertility soils have a CEC <5 cmol+/kg.
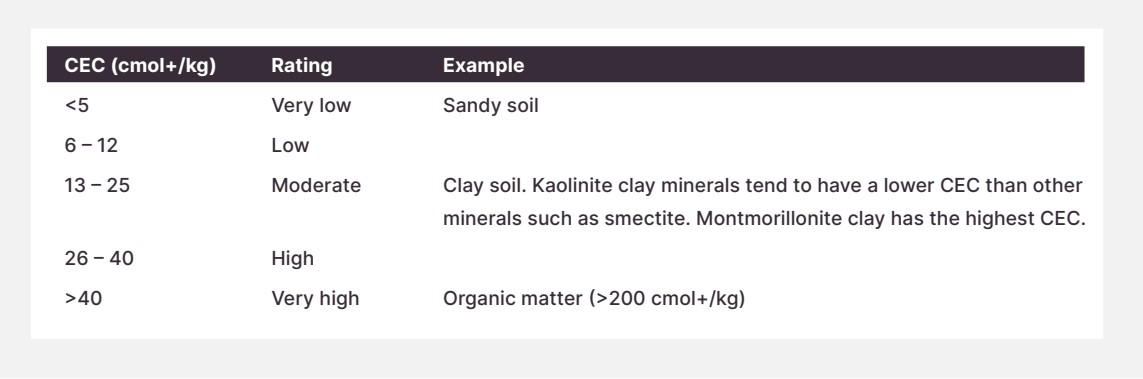
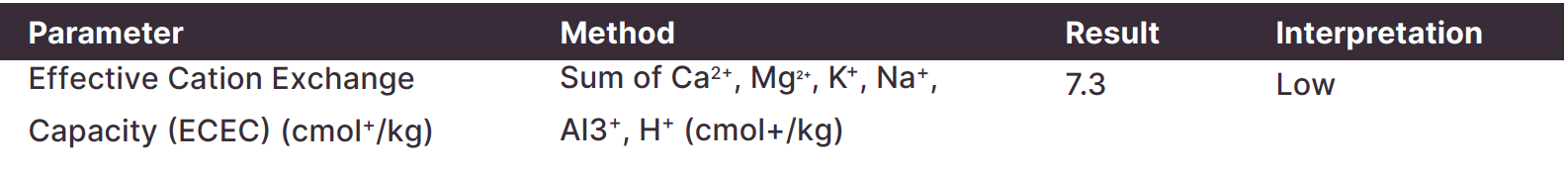
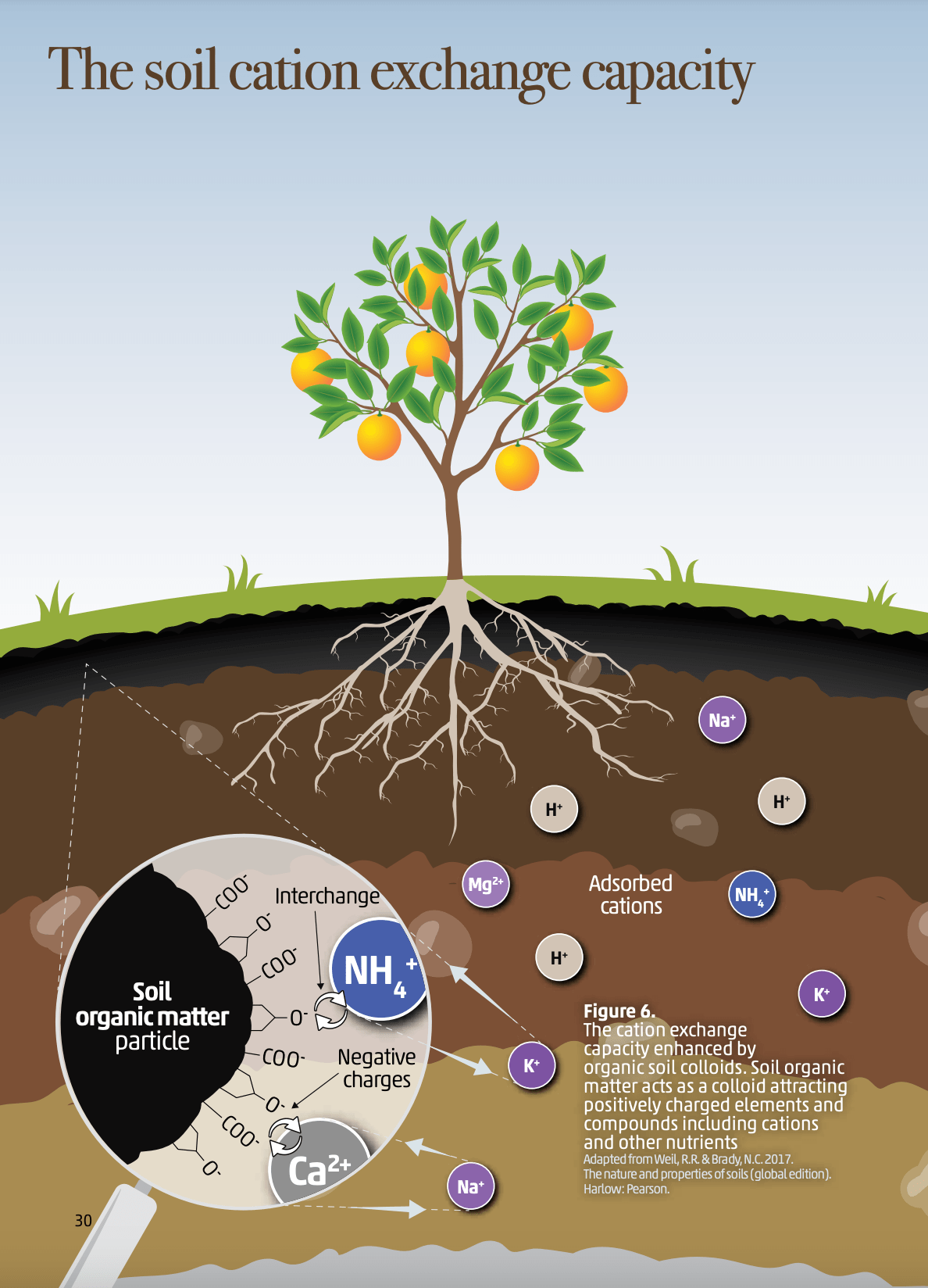
Figure 3: ’The soil cation exchange capacity' in "Soils for Nutrition: State of the Art" by the Food and Agriculture Organization of the United Nations (FAO), 2022.
The ideal balance of exchangeable cations is debated in soil science circles. Sometimes an ideal range is listed, which is about 15-25% Mg2+, 5 – 15% K+, >50% Ca2+and <6% Na+. It’s best not to focus too much on cation ratios, except when looking at exchangeable sodium, exchangeable aluminium, and the calcium to magnesium ratio.
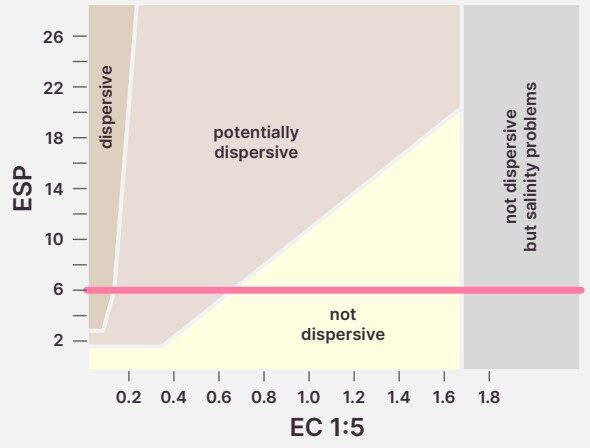
A soil with an exchangeable sodium percent (ESP) >6 is considered sodic. This means the soil could be dispersive. Dispersive soils collapse in water, and dry into a hard, structureless mass. However, ESP alone is not enough to gauge dispersion, as it is affected by soil salinity and other soil factors. For example, soil salinity suppresses dispersion (See Figure 4).
If ESP is > 5, it is worth checking if your soil is dispersive. This is best done by an ASWAT or EAT test (See section on Soil Dispersion). ESP is useful to see sodium itself is hindering crop root growth, and to calculate liming rates if the soil is dispersive.
Very acidic soils can have high exchangeable aluminium (Al3+) which is toxic to plant roots. Exchangeable aluminium only becomes an issue when pH in water drops below 5.5. Exchangeable aluminium levels >1% could indicate a problem for sensitive species. Aluminium is an issue for sensitive crops when levels >2 mg/kg.

Many soil test results report the Ca2+: Mg2+ ratio as an indicator of soil stability. This ratio is best used as a general guideline when troubleshooting as soils can have a wide range of cation ratios with little impact on soil structure:
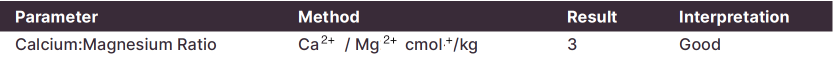
The Ca:Mg ratio of this soil is >2, This value, coupled with the low ESP (1.7%) suggest soil stability or dispersion are not an issue.
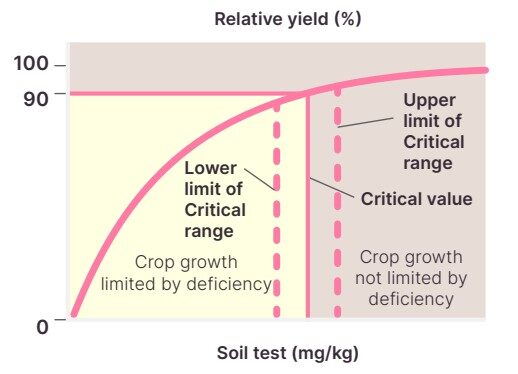
Nutrient results need to be interpreted for each situation. Determining whether a result is low or high depends on the critical value for the specific nutrient.
The critical value is the number required to achieve 90% of the yield potential (See Figure 5) and will vary depending on the soil, climate, crop, and growth stage. If the test result is above the critical range, the crop probably won’t respond to additional nutrients. Test results below the critical value indicate crop growth is limited by nutrient deficiency and will respond to applied nutrients (See Figure 5).
See Case study 1 and 2 to for an example on how to interpret test results according to critical values.
The three main forms of crop available nitrogen in the soil are nitrate-N (NO3-), ammonium-N (NH4+) and organic N. Nitrate and ammonia are the easiest for plants to use. Generally, nitrogen needs to be mineralised from organic matter or applied in a readily available form for crops to use it.
Organic N and ammonium-N are not very mobile in the soil. 0 – 10cm soil tests tend to gauge these numbers well. Nitrate-N is mobile and better assessed with deeper soil testing.
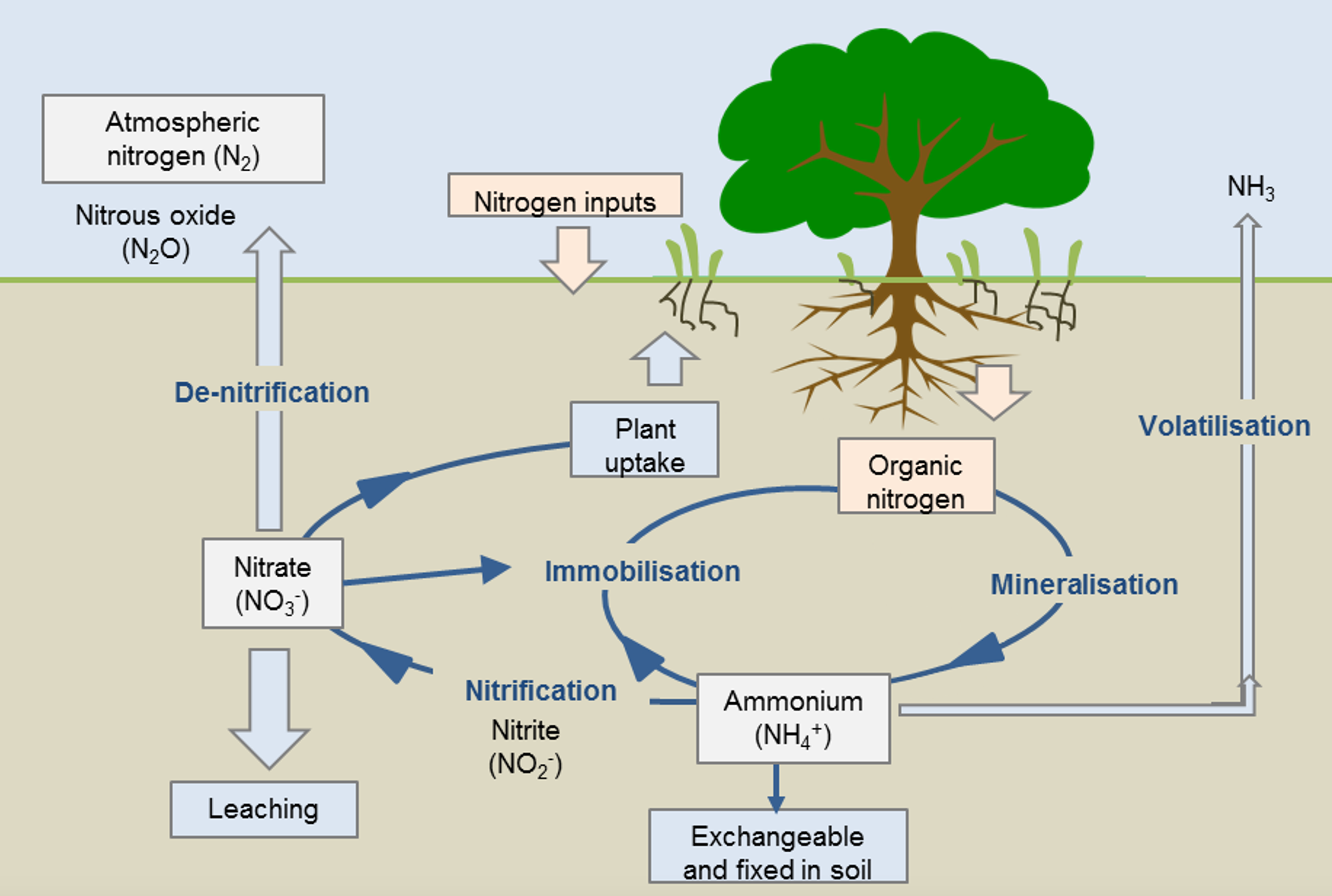
Figure 6. Soil nitrogen (N) cycle. The cycling of N through different forms in the soil determines the quantity of N that is made available for plant uptake.
Source: Agriculture WA.
The common laboratory tests are total nitrogen, ammonium, and nitrate-N. Laboratories often have their own test methods.
Total nitrogen is the total amount of N in the soil. Most of it will be bound up in organic matter (the organic N) and not readily available for crops to use. Total N is not a guide to the amount of available N. The result is used to calculate the carbon:nitrogen ratio (C/N ratio).
Nitrate-N and ammonium-N are presented as mg/kg or ppm.
Nitrate-N and ammonium-N can vary considerably in the soil. Need local experience to interpret the results, as they depend on soil moisture, temperature, time of sampling. Generally, if nitrate-N is < 5 mg/kg, the value is considered low.
Total-N is often tested with the LECO (as with testing organic carbon) and results are given as a %.

Soil test results sometimes present a C:N ratio. This ratio can significantly impact crop residue decomposition affecting residue cover on soil and crop nutrient cycling, predominantly N. It is important to understand C:N ratio of different crop residues when planning cash crops rotations and cover crops in rotations.
For organic materials, when the C:N ratio is above about 20:1 (20 times more C than N), decomposition slows. In broadacre cropping, this can mean growers need to apply N fertiliser to help stubble breakdown, otherwise soil microbes will use soil N stocks to do their work, temporarily creating an N deficit.
Soils with C:N ratio of 24:1 is the best for soil microbes to make nutrients like N, P and Zn available to plants.
Phosphorus in soil is in several forms such as Available P (PO43-), quantity P (bound) and diffusional P. Quantity P is the component of P off soil solid phases that may be potentially available during the course of the life of the plant when the intensity pool is depleted by plant uptake. Phosphorus availability is highly limited factors such as pH.
There are four main phosphorus tests. It is important to choose the right test for your situation. Phosphorus moves in and out of different pools in the soil and the different tests can measure different pools of P.
The different test methods extract varying amounts of P from the different pools. Most tests measure readily available P plus some organic/inorganic P which becomes available over the season.
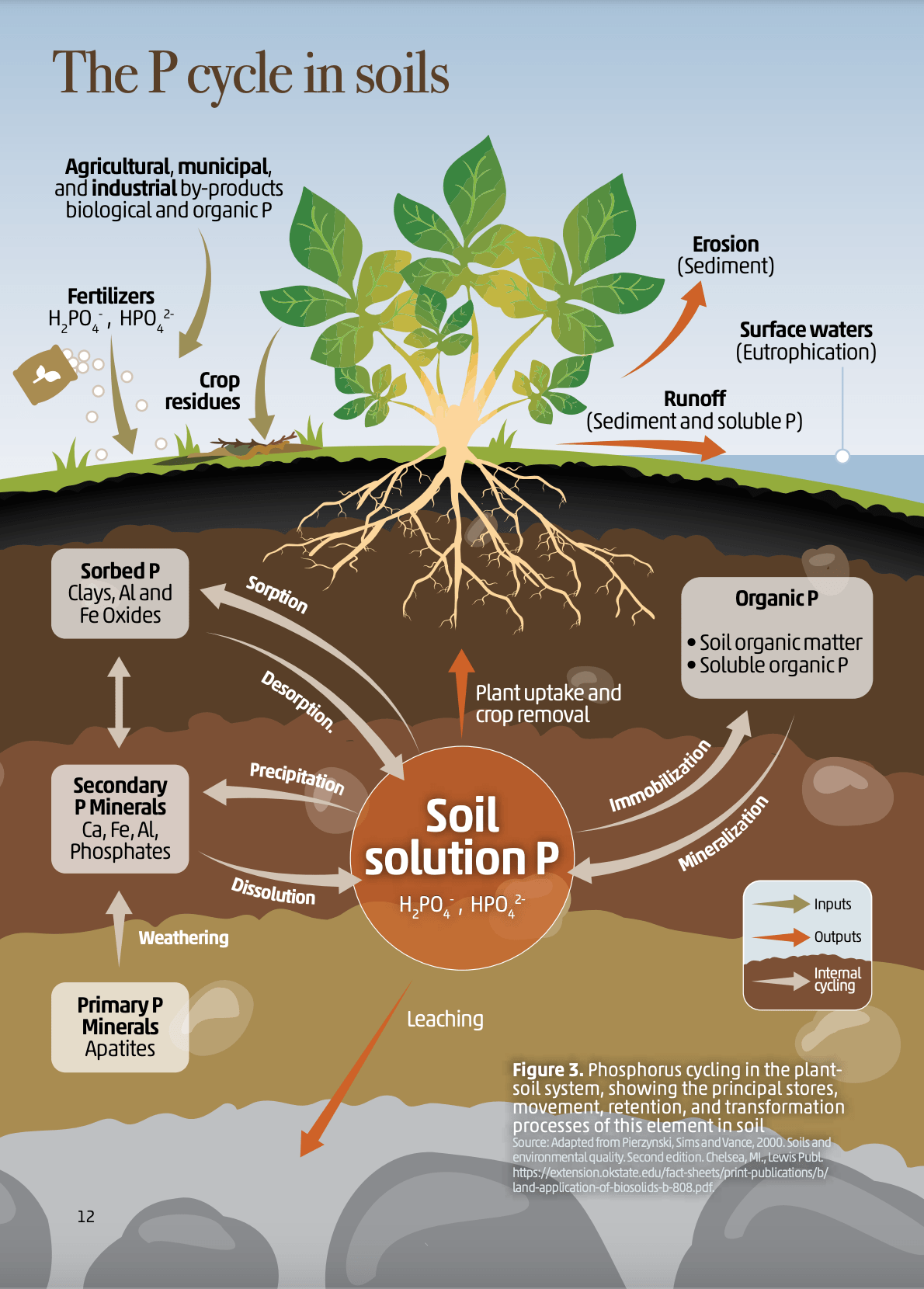
Figure 7: The P Cycle in Soils’ in "Soils for Nutrition: State of the Art" by the Food and Agriculture Organization of the United Nations (FAO), 2022.
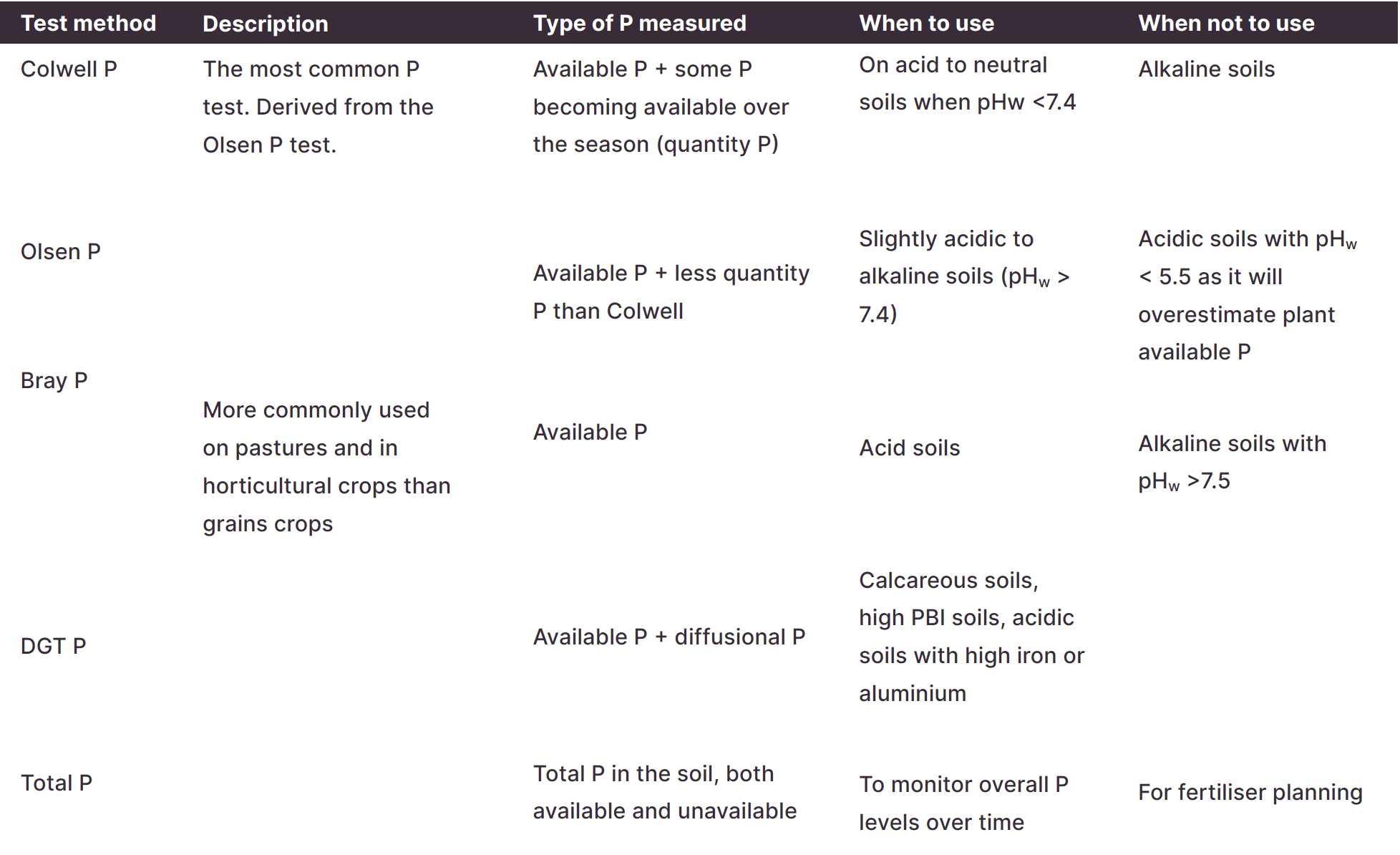
Colwell P test results should be interpreted with the phosphorus buffering index (PBI), which describes the ability of a soil to ‘lock up’ P (see PBI below).
The critical value will depend on the crop, location, and P test used for Colwell, Olsen and Bray methods. As the DGT-P test is still relatively new, there are fewer interpretation guidelines.

The phosphorus buffering index (PBI) describes the ability of soil to ‘lock up’ or immobilise phosphorus. The higher the PBI, the more P it will lock up which affects P fertiliser application rates.
P quickly binds on exchange sites on higher PBI soils, making it unavailable for plants to use. Soils with a low PBI do not lock up much P, meaning a greater risk of P leaching.
Higher PBI soils need more P fertiliser. Over time the exchange sites are ‘filled’ and more P is available for crops to use.
Table below shows PBI and Colwell P critical values for pastures, wheat, and potatoes. The critical value is the Colwell P soil test value needed to achieve 90% yield with the respective PBI.
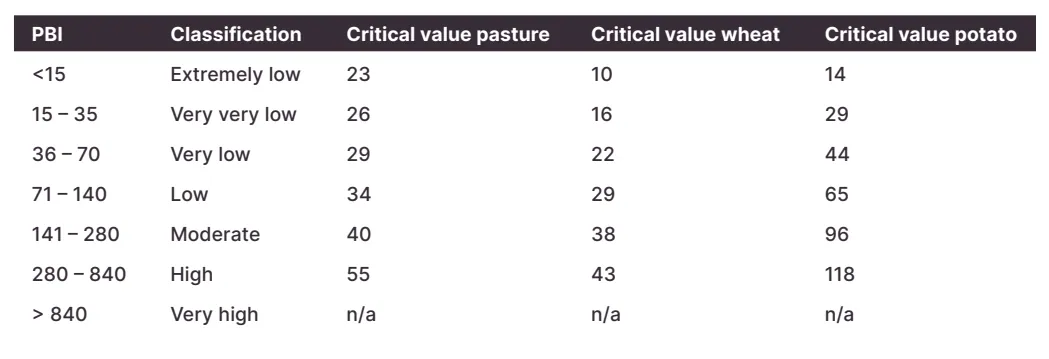
Reference: Wheat- Moody, 2007; Potato - APAL
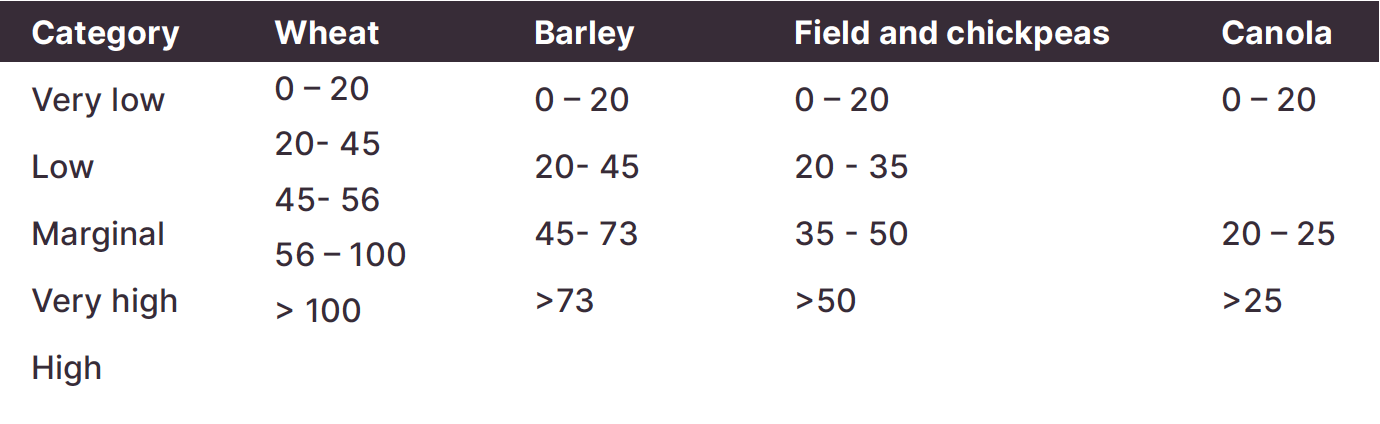
A pasture on a soil with a PBI of 80, needs a Colwell P value of at least 34 mg/kg. Potatoes on the same soil need 65 mgP/kg.
Available potassium is measured as either extractable K (Colwell-K) or exchangeable K. Colwell-K usually has higher results because the test measures exchangeable K+ + water soluble K+ + a small amount of fixed K.
On sandy soils, there is a rough relationship where exchangeable K+ x 391 = Colwell-K.
If CEC has been tested, the results should list exchangeable K.
To convert between cmol+/kg and mg/kg:
mg/kg of extractable or exchangeable K+ = 391 x cmol+/kg
Use the following as general guidelines only to see if test results are significantly higher or lower than these values. For proper crop nutrition, use critical values suited to your region, soil type and specific crop. For example Lucerne has higher K demands. Also, K demand increases with clay content.


Only exchangeable potassium was measured on this soil sample. The result, 430 mg/kg is well above the general guidelines given above, indicating potassium deficiency is not an issue on this soil at the moment.
Most sulphur tests measure the sulphate form of S, the form plants take up. The two common tests are KCl-40 and MCP.
The KCl-40 test is commonly used as it takes into account the available S and some of the S that will become available when organic matter mineralizes over the season. It is considered more accurate because it tests these two pools. For soils high in organic matter, request the KCl-40 test. This test tends to be more reliable on sandier soils but can give low readings after long, dry periods or leaching rains.
The MCP test does not sample the organic pool of S and can underestimate S supply.

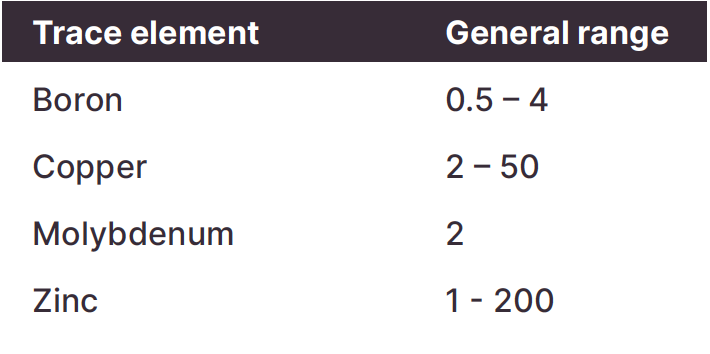
Trace elements are usually tested using the DTPA method, but this method is best used in neutral to alkaline soils. The EDTA method is more accurate in acid soils.
Soil testing is not very reliable to gauge plant available nutrients but is better used to track overall nutrient stocks. Plant tissue testing is a better gauge of trace element deficiencies and toxicities than soil testing. When it comes to trace elements it’s better to ask the plant directly with tissue testing than infer from soil test results.
As with the macro nutrients, critical levels will vary between soil types and plants.
Things to look out for:
Levels outside those in the table might warrant a closer look.
Related tests: exchangeable sodium percent and salinity. Dispersive soils tend to have elevated (>6%) levels of exchangeable sodium. Salinity can suppress dispersion.
Dispersion testing can be done at home, or samples can be sent to the lab. Air-dried peds are placed into a dish of deionised water, and dispersion and slaking are measured after 10 minutes, 2 hours, and 24 hours. Methods vary slightly depending on whether the lab is using the Emerson Aggregate Test or the ASWAT Test.
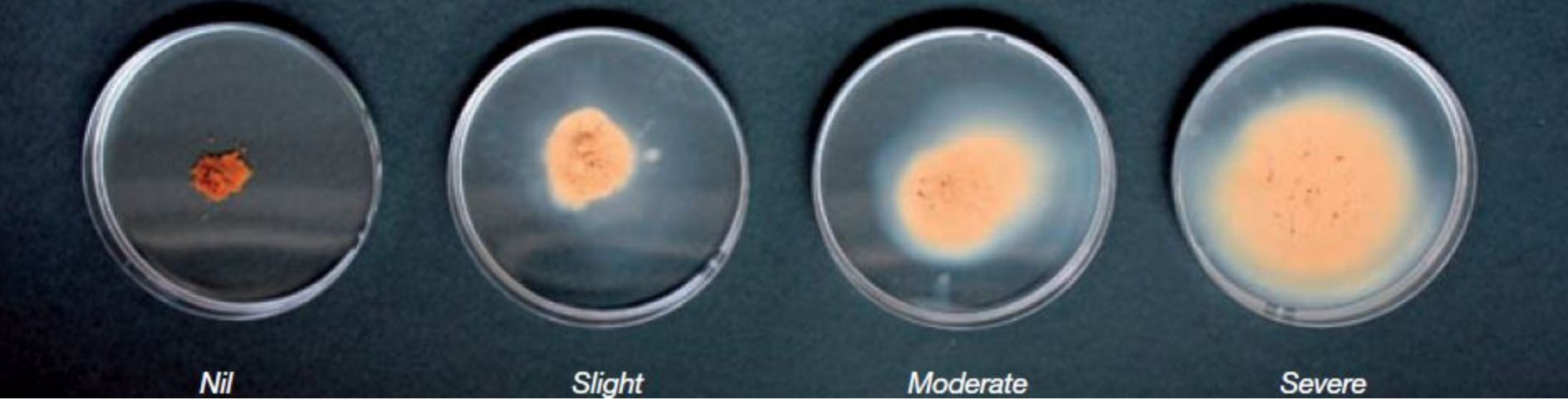
Figure 8. A simple soil dispersion test - DPIRD WA
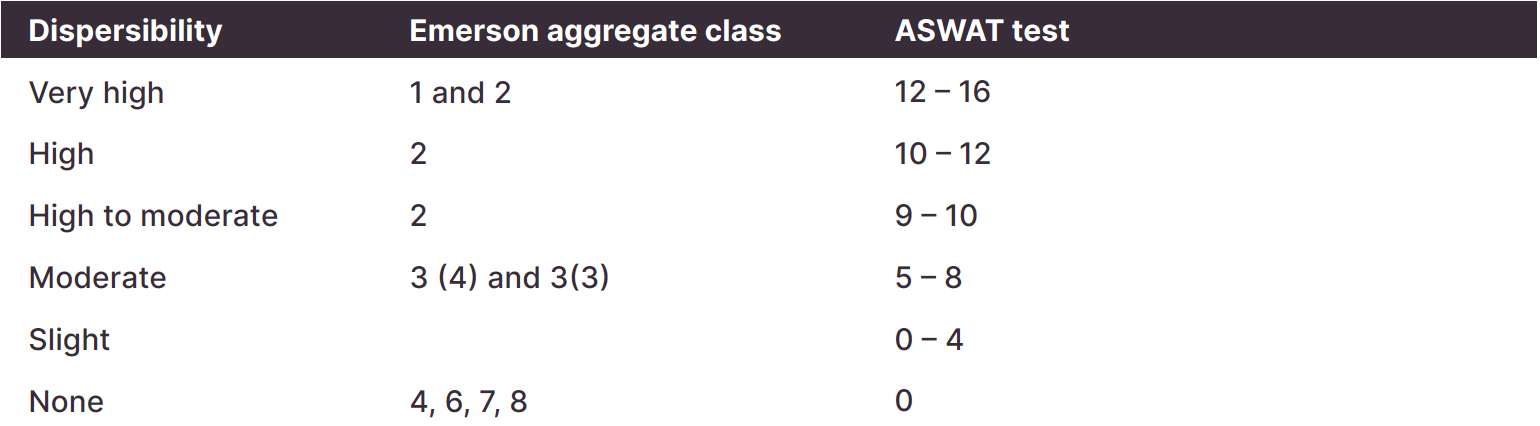
Sandier soils tend to have lower organic matter, hold less water and nutrients, and are prone to water repellence. Sandier soils usually benefit from split nutrient applications as they are more prone to leaching, particularly of N, K and S. Clay soils are more fertile but can have structural problems (high soil strength, dispersion) and are more prone to waterlogging.
Soil colour is a guide to minerals and drainage. Iron plays a decent role in soil colour. In well-drained soils, iron Is red. As oxygen levels decrease, iron becomes more yellow.
Brighter colours indicate better drainage.
Regularly waterlogged soils tend to have grey, green or even blueycolours.
Soil mottles, patches of different colour, indicate the soil is periodically waterlogged.

If monitoring soil properties, the results will fluctuate over time with the season and soil moisture conditions. Many soil properties are affected by seasonal changes, including pH, soil carbon, bulk density and nutrients. Collecting samples at the same time each year helps with consistency.
For an example, pHw tends to be lower in drier conditions, with values fluctuating by up to 0.6 pH units in a year. Soil pHCaCl2 is less influenced by seasons.